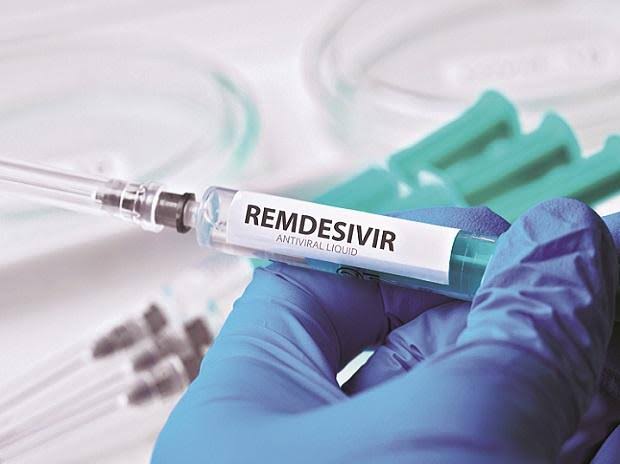
The pharmaceutical frontrunner Gilead Sciences had released their Remdesivir formulation in May. Following this, it was said that they were in talks with major pharmaceutical companies in Asia for mass production of the same. In accordance with the same, the Union Health ministry of India has given the mod to Hetero and Cipla to produce Remdesivir as a generic medicine.
Gilead Sciences had applied to the Indian Drug Regulatory Agency, CDSCO, for import and marketing of Remdesivir on May 29. After due deliberations, permission under emergency use authorization was granted by DCGI on June 1 in the interest of patient safety and obtaining further data.
The two companies have been granted permission to use the formulation to produce and market the antiviral drug Remdesivir for “restricted emergency use” on hospitalised COVID-19 patients.
This decision has been a result of the unmet medical necessities of those affected by the Coronavirus infection. The Drug Controller General of India (DCGI) has fastracked this approval process after noticing the rapid increase in number of cases reported. This approval has come only a day after Glenmark Pharmaceuticals had gotten their antiviral drug approved.
Meanwhile, India’s coronavirus toll has crossed 4,00,000 while the number of deaths stand at 13,281.