Tamil Nadu Health Minister Ma. Subramanian on Friday (17 October 2025) seemed to absolve his government of responsibility in the deaths of 25 children in Madhya Pradesh linked to Coldrif cough syrup manufactured in Tamil Nadu by Sresan Pharmaceuticals, claiming that the incident was caused by “negligence on the part of Madhya Pradesh drug quality control authorities.”
Responding to a special motion raised by Opposition Leader Edappadi K. Palaniswami in the Tamil Nadu Assembly, Subramanian said that the state acted “within hours” of receiving information from Madhya Pradesh about the contaminated “Coldrif” syrup. He maintained that Tamil Nadu’s role was limited to regulation and that the company involved, Sresan Pharmaceuticals, had already been sealed and its operations halted.
Minister’s Explanation in the Assembly
The minister stated that the Tamil Nadu Drugs Control Department received an official communication from its Madhya Pradesh counterpart at 3 PM on 1 October 2025, reporting the death of a child in Chhindhwara district after consuming Coldrif syrup. The letter also contained details of the chemical substances suspected to have caused the fatality.
According to Subramanian, the Deputy Director of Drug Control immediately directed a senior inspector to conduct a spot inspection of the manufacturer. “By 4 PM the same day, inspections were initiated,” he said, asserting that the state government “was not a buyer of medicines” from the company. He also claimed that within 30 minutes of receiving the information, the sale of the cough syrup was banned across Tamil Nadu, and orders were issued preventing private retailers from stocking or distributing it.
Violations Found; 126 Drugs Suspended
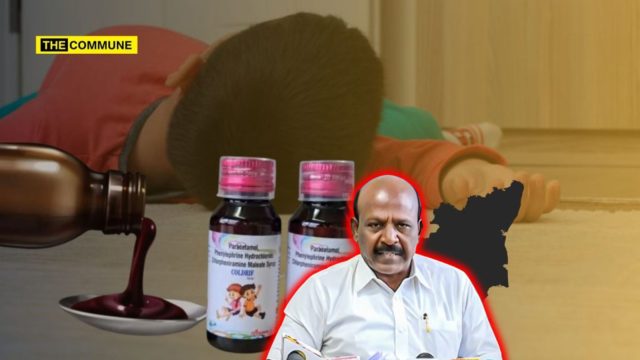
Ma Subramanian stated that inspections carried out on October 1 and 2 reportedly revealed multiple violations in the company’s operations. Laboratory analysis of five medicines, including Coldrif, showed the presence of 48.6% diethylene glycol, a toxic chemical known to be life-threatening. Subramanian confirmed that the company’s entire range of 126 products was suspended, and a “stop production order” was issued on 3 October 2025.
He stated that Coldrif syrup had also been distributed in Puducherry and Odisha, and that Tamil Nadu authorities immediately alerted those states to prevent further tragedies. “The company was sealed, and a notice was issued to explain why its licence should not be revoked. Criminal action has been initiated against its owner, Ranganathan, who has been arrested,” he told the House.
Blames MP Officials, Says TN Acted Responsibly
While asserting that the Tamil Nadu government took “swift and comprehensive action,” Subramanian argued that the actual responsibility for the deaths lay with Madhya Pradesh’s regulatory mechanism. “The deaths occurred because of negligence by the drug quality control officials in that state,” he said, adding that Tamil Nadu had fulfilled its duty by taking immediate preventive steps.
Subramanian claimed that Chief Minister M.K. Stalin had instructed officials to pursue criminal proceedings against the company but also cautioned that political motives should not be allowed to affect India’s image in the global pharmaceutical market. “Our pharmaceutical industry exports drugs worth ₹12,000 to ₹15,000 crore annually. Inspections are being conducted continuously across all 397 manufacturing units,” he said.
Following the investigation, the Sresan Pharmaceuticals factory was permanently closed, and its licence suspended. “The plant is now completely shut down,” the minister confirmed, noting that the company had been barred from producing or distributing any medicine pending legal proceedings.
Subramanian concluded his statement by urging the public to avoid consuming any medicine without a doctor’s prescription and reiterated that Tamil Nadu’s regulatory system had acted responsibly once the alert came from Madhya Pradesh.
மத்திய பிரதேசத்தில் கோல்ட்ரீஃப் இருமல் மருந்து குடித்து குழந்தைகள் இறந்ததற்கு யார் காரணம்..? சட்டப்பேரவையில் அமைச்சர் மா.சு விளக்கம்#TNAssembly | #MinisterMaSubramanian | #ColdRif | #CoughSyrup | #PolimerNews pic.twitter.com/jxNbxiAqy5
— Polimer News (@polimernews) October 17, 2025
The opposition, however, accused the DMK government of deflecting blame and failing to proactively monitor pharmaceutical manufacturers operating within the state.
(Source: OneIndia Tamil)
Subscribe to our channels on Telegram, WhatsApp, and Instagram and get the best stories of the day delivered to you personally.