The Drugs Controller General of India (DGCI) approved for restricted use in emergency situations two vaccines – indigenous COVAXIN brought out by Bharat Biotech and Oxford Institute’s Covishield which is being developed by Pune-based Serum Institute.
DGCI VG Somani said at a media briefing that “we will never approve anything if there is slightest of safety concern.”
“The vaccines are 110% safe. Some side effects like mild fever, pain & allergy are common for every vaccine”, the DGCI said while rubbishing the claims of vaccines making people impotent.
The DGCI also said that Covishield was found to be 70.42% effective and Bharat Biotech’s Covaxin was “safe and provides a robust immune response”.
In a statement issued by Bharat Biotech, it noted that 23,000 volunteers have been received for phase 3 trials and was progressing towards achieving the goal of 26,000 participants. The statement also said that it is the largest phase 3 efficacy trial ever conducted for any vaccine in the country.
However, Congress MP from Thiruvananthapuram Dr. Shashi Tharoor in a tweet claimed that COVAXIN has not yet had phase 3 trials and stoked fears that the approval for the vaccine could be dangerous.
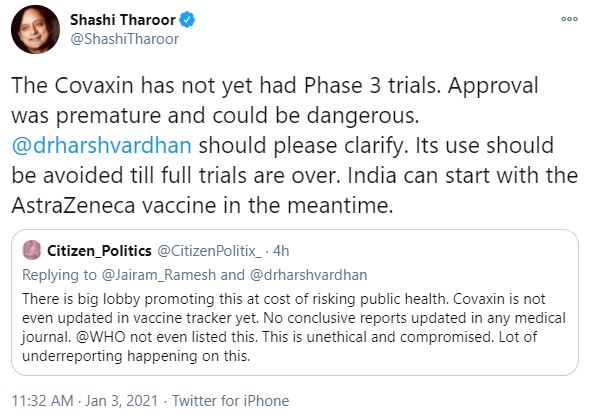
It is to be noted that human clinical trials of Bharat Biotech’s COVAXIN began in mid November. The data on phase 3 trials conducted so far has already been submitted to DGCI.